Online TestScience
Atoms and Molecules
Atoms and Molecules
Congratulations - you have completed Atoms and Molecules.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Your answers are highlighted below.
Question 1 |
100 ml of oxygen gas and 100 ml of nitrogen gas at normal temperature and pressure contain ................
equal number of atoms | |
equal number of molecules | |
oxygen gas has more number of atoms than nitrogen gas | |
nitrogen gas has more number of atoms than oxygen gas |
Question 2 |
Identify the monatomic and diatomic species from the following pairs .............
helium and neon | |
hydrogen and chlorine | |
chlorine and ozone | |
helium and oxygen |
Question 3 |
The molecular mass of chlorine is 71 and its atomic mass is 35.5. The atomicity of chlorine is ........................
1 | |
2 | |
3 | |
cannot be predicted |
Question 4 |
the atomic mass of sulphur is 32. It has atomicity 8. The molecular mass of sulphur is .............
32 | |
256 | |
4 | |
42 |
Question 5 |
Isobars are ..................
atoms of the same element with same atomic number but different mass number | |
atoms of different elements having the same mass number but different atomic number | |
atoms of different elements with the same number of neutrons | |
atoms of different elements having the same atomic number and different mass number |
Question 6 |
6C14 and 7N14 are examples of ........................
isotopes | |
isobars | |
isotones | |
isosters |
Question 7 |
Which of the following statement truly reflect the definition of atom and molecule?
atoms are unstable and do not exist free whereas molecules exist free | |
atoms of polyatomic molecules do not exist free whereas molecules exist free | |
atoms of all types of molecules and molecules exist free | |
monoatomic elementary molecule and molecules do not exist free |
Question 8 |
Which of the following have independent existence?
an atom of nitrogen | |
an atom of helium | |
a molecule of chloride | |
both (ii) and (iii) |
Question 9 |
Which of the following pairs constitute an elementary molecule?
hydrogen and chlorine | |
water and nitric oxide | |
methane and water | |
Hydrogen and water |
Question 10 |
Identify the pair which consists of a homoatomic molecule and a hetero atomic molecule ................
- nitrogen and ammonia
- nitrogen and oxygen
- oxygen and water
- chlorine and nitrogen
1 and 2 | |
1 and 3 | |
3 and 4 | |
1 and 4 |
Question 11 |
Which of the following has highest mass in grams?
1 atom of silver | |
1 mol of nitrogen | |
1 mol of calcium | |
2 g of sodium |
Question 12 |
Which among the following will contain the same number of atoms of oxygen and other element in one mole of that substance .......................
- carbon monoxide
- nitrous oxide
- nitrogen dioxide
- carbon-dioxide
1 and 2 | |
3 and 4 | |
1 and 3 | |
2 and 4 |
Question 13 |
The volume occupied by 2 mol of nitrogen dioxide at STP is ..................
22.41 | |
44.821 | |
2.2421 | |
4.4821 |
Question 14 |
One mole of the oxygen gas has the volume of ........................
1 L of oxygen a STP | |
32 L of oxygen a STP | |
22.4 L of oxygen a STP | |
none |
Question 15 |
How many moles are represented by 36g of water?
1 | |
2 | |
3 | |
4 |
Question 16 |
What is the mass of 4.48 x 10-2m3 of Methane gas at STP?
16 g | |
32 g | |
48 g | |
54 g |
Question 17 |
Gram molecular mass of nitrogen is ....................
23 | |
14 | |
7 | |
none |
Question 18 |
The gram-atomic mass of chlorine is ......................
35.5 g | |
35.5 kg | |
3.55 g | |
3.55 kg |
Question 19 |
4.25 g of ammonia is equal to ....................
0.25 mole | |
1 mole | |
1.5 mole | |
0.5 mole |
Question 20 |
Which of the following will contain the mass number of atoms as 20g of calcium?
24 g of Mg | |
12 g of C | |
24 g of C | |
12 g of Mg |
Question 21 |
Avogadro number of helium atoms weigh .....................
1.00 g | |
4.00 g | |
8.00 g | |
4 x 6.023 x 1023g |
Question 22 |
The mass of 2.24 dm3 of a gas under standard condition is 2.8 g. Its molar mass is .....................
28 | |
14 | |
42 | |
56 |
Question 23 |
Atoms of the same elements having similar chemical properties and different physical properties are known as ..................
isotopes | |
isobars | |
isotone | |
isochors |
Question 24 |
The species that take part in chemical reactions are ..................
atoms | |
molecules | |
electrons | |
protons |
Question 25 |
The ratio of atoms in a molecule of H2SO4 is ..................
2:1:4 | |
2:2:4 | |
1:1:1 | |
2:2:3 |
Question 26 |
.................. is the name given to a process where atoms of one element is changed to atoms of another element.
disintegration | |
transmutation | |
atomisation | |
combination |
Question 27 |
Tri atomic molecules contain ......................... number of atoms.
three | |
four | |
two | |
six |
Question 28 |
The molecular mass of an atom is 32 and its atomic mass 8. Its atomicity is ..................
4 | |
8 | |
2 | |
3 |
Question 29 |
The atomicity of a molecule is 3. Its atomic mass is 16. Its molecular mass is .............
48 | |
16 | |
32 | |
18 |
Question 30 |

A | |
B | |
C | |
D |
Question 31 |

1 and 2 | |
2 | |
2 and 4 | |
3 and 4 |
Question 32 |
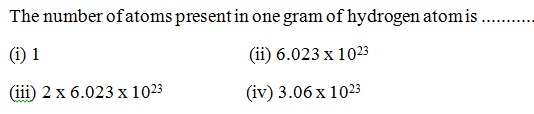
A | |
B | |
C | |
D |
Question 33 |

A | |
B | |
C | |
D |
Question 34 |
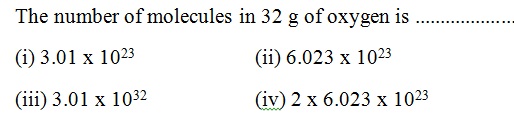
A | |
B | |
C | |
D |
Question 35 |

A | |
B | |
C | |
D |
Question 36 |
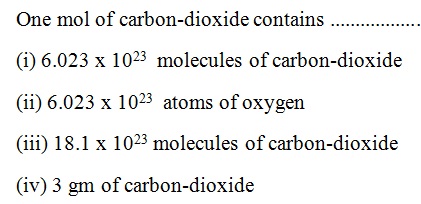
A | |
B | |
C | |
D |
Question 37 |

A | |
B | |
C | |
D |
Question 38 |
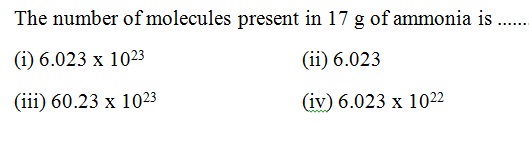
A | |
B | |
C | |
D |
Question 39 |

A | |
B | |
C | |
D |
Question 40 |

A | |
B | |
C | |
D |
Question 41 |

A | |
B | |
C | |
D |
Question 42 |
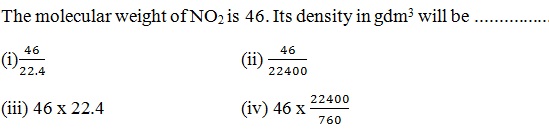
A | |
B | |
C | |
D |
Question 43 |
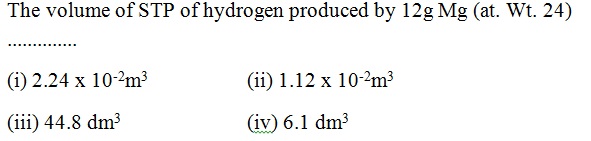
A | |
B | |
C | |
D |
Question 44 |

A | |
B | |
C | |
D |
Once you are finished, click the button below. Any items you have not completed will be marked incorrect.
There are 44 questions to complete.